ISO 13485 Medical Devices Quality Management System Certification
Introduction of ISO 13485
ISO 13485 is an internationally recognized standard that outlines specific quality management system (QMS) requirements for organizations engaged in various aspects of the medical device industry, including the design, production, installation, servicing, and manufacturing of medical devices.
First published in 1996 and subsequently updated in 2003 and 2016, ISO 13485 continues to evolve to address the dynamic nature of the healthcare sector. Compliance with ISO 13485 is essential for organizations looking to demonstrate their commitment to producing safe and effective medical devices, adhere to stringent regulatory requirements, and maintain the highest levels of quality and patient safety.
What Is ISO 13485 Certification?
The ISO 13485 Certification is a harmonized ISO standard, which lays-down the needs for quality management systems for medical devices.
ISO 13485 Certifications offers manufacturers, suppliers and designers to the medical-device industry with a management system necessary to demonstrate compliance to regulatory need and mitigate hazard to stake-holders. It places more emphasis on hazard based thinking and decision making while it also provide stronger interoperability between the clauses & requirements.
Key Significance
- Boost the export advantage
- Clarification of Management Responsibilities
- ISO 13485 Boost customer satisfaction
- By help of ISO 13485 Certification integrate Risk Management
- ISO 13485 assures for good quality products and services
- Standard makes sure patient safety
Who Requires ISO 13485 Certification?
ISO 13485 certification is required for designers, suppliers and manufacturers of medical devices. However, ISO 13485 certification is relevant for a wide range of stakeholders in the medical device industry, including manufacturers, suppliers, regulatory authorities, healthcare institutions, and testing organizations and gain a competitive advantage by enhancing the organization brand’s credibility and recognition.
ISO 13485 certification is required by law in some countries like Australia, European Union, Japan, New Zealand, Russia, Singapore, South Korea, United States etc and it is also a requirement for many government and private sector contracts. ISO 13485 certification is relevant for a wide range of stakeholders in the medical device industry, including manufacturers, suppliers, regulatory authorities, healthcare institutions, and testing organizations.
Why choose SQC
- Expertise
- Reputation
- Global Recognition
- Client Centric Approach
- Results Driven
- No Middlemen
Choosing SQC Certification Services as your certification body ensures that you receive expert assessments and a streamlined certification process that paves the way for your organization to achieve ISO 13485:2016 certification.
Cost of ISO 13485:2016 Certification
Understanding the costs associated with ISO 13485:2016 certification is essential for organizations considering this endeavor. The cost of certification can vary depending on several factors, including the size of your organization, the complexity of your processes, and the Certification Body (CB) you choose. To know more about ISO 13485 certification cost, contact us.
Conclusions
By selecting SQC Certification Services as your certification body, you’re taking a significant step towards quality excellence, operational efficiency, and sustainable growth in the medical device industry.
Our Services
ISO 13485:2016 Certification - Elevating Medical Quality Assurance
Welcome to our ISO 13485:2016 Certification page, where we delve into the significance, advantages, and processes of achieving this critical certification, specifically tailored for the medical device industry.
Understanding ISO 13485:2016 Certification
ISO 13485:2016 is a globally recognized standard specifically designed for organizations involved in the design, development, production, installation, and servicing of medical devices. It sets stringent requirements for a Quality Management System (QMS) tailored to the unique needs of the medical device industry.
Why is ISO 13485:2016 Certification Crucial?
For organizations in the medical device sector, patient safety and product quality are paramount. ISO 13485:2016 Certification signifies a commitment to adhering to the highest standards of quality and regulatory compliance. It not only fosters confidence among customers and stakeholders but is often a prerequisite for market access.
ISO 13485:2016 Certification Process
Becoming ISO 13485:2016 Certified is a meticulous process designed to ensure the highest levels of quality and safety. It includes:
- Gap Analysis: We begin with a thorough evaluation of your existing quality management practices, identifying areas that require alignment with ISO 13485:2016 standards.
- QMS Development: Together, we craft a robust Quality Management System that integrates ISO 13485:2016 requirements into your daily operations.
- Adoption: The QMS is put into action, encompassing quality control measures, risk management, traceability, and compliance checks.
- Internal Audits: Periodic internal audits assess the effectiveness of your QMS, offering insights for continual improvement.
- Certification Audit: An accredited certification body conducts a final audit to determine if your organization complies with ISO 13485:2016 standards.
What is the Requirements of ISO 13485:2016 Certification
- Establishment of a Quality Management System (QMS) specific to medical devices.
- Documented processes and procedures for design, development, production, and servicing.
- Implementation of risk management processes throughout the product lifecycle.
- Comprehensive record-keeping and documentation management.
- Rigorous product and process validation and verification.
- Traceability of all devices, components, and materials.
- Effective complaint handling and reporting systems.
- Robust corrective and preventive action processes.
- Thorough evaluation and control of suppliers and outsourced processes.
- Adherence to regulatory and statutory requirements.
- Comprehensive training programs for personnel.
- Ongoing monitoring, measurement, and analysis of QMS performance and product quality.
ISO 13485:2016 Certification Principles
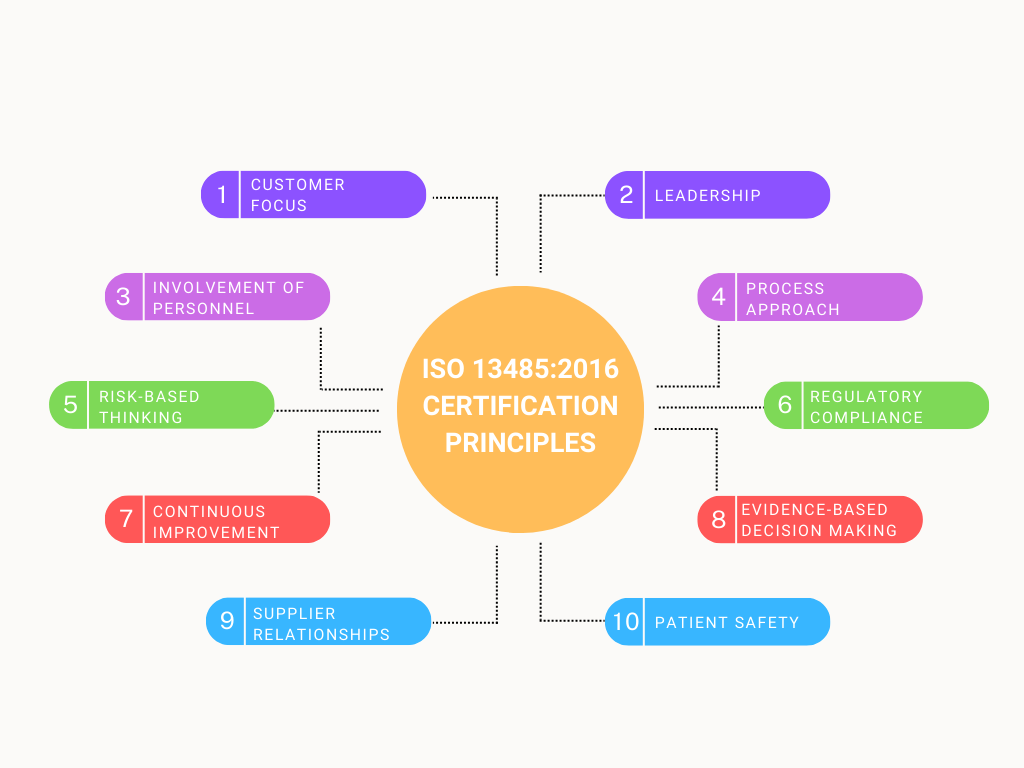
ISO 13485 Certification is built upon several key principles that guide organizations in the medical device industry towards the development and maintenance of a robust Quality Management System (QMS) focused on product quality and patient safety. These principles include:
Customer Focus: ISO 13485 emphasizes meeting customer and regulatory requirements. Organizations must understand the needs and expectations of their customers and stakeholders, particularly in terms of medical device quality and safety.
Leadership: Top management plays a pivotal role in establishing and maintaining a culture of quality and regulatory compliance within the organization. Leaders are responsible for setting quality objectives and ensuring that the QMS is effectively implemented and continuously improved.
Involvement of Personnel: ISO 13485 recognizes that the involvement and competence of personnel at all levels are critical to the success of the QMS. Organizations must ensure that their staff is adequately trained and aware of their roles in maintaining quality and safety.
Process Approach: The standard promotes a process-oriented approach to quality management. Organizations are encouraged to identify, define, and manage key processes that contribute to product quality and regulatory compliance.
Risk-Based Thinking: ISO 13485:2016 introduced a more prominent focus on risk management. Organizations are required to identify and assess risks associated with their products and processes and implement measures to mitigate or control these risks effectively.
Regulatory Compliance: ISO 13485 places significant importance on compliance with regulatory requirements. Organizations must ensure that their QMS aligns with applicable medical device regulations and standards in the regions where they operate.
Continuous Improvement: A commitment to continual improvement is a fundamental principle of ISO 13485 Certification. Organizations are expected to regularly evaluate their processes, products, and the effectiveness of their QMS. Any identified opportunities for improvement should be addressed promptly.
Evidence-Based Decision Making: Organizations are encouraged to base their decisions on data and evidence. This principle emphasizes the importance of data analysis in monitoring and improving product quality and safety.
Supplier Relationships: ISO 13485 recognizes the significance of supplier relationships. Organizations must ensure that their suppliers and subcontractors meet quality and regulatory requirements to maintain the integrity of the supply chain.
Patient Safety: Patient safety is at the core of ISO 13485 Certification. Organizations must design and manufacture medical devices with a primary focus on ensuring the safety and well-being of patients.
These principles collectively provide a framework for organizations in the medical device industry to establish, maintain, and continuously improve their QMS, resulting in the production of safe and high-quality medical devices that meet regulatory requirements and customer expectations.
A Commitment to Continuous Improvement
ISO 13485:2016 Certification signifies a dedication to continuous improvement. By maintaining and continually enhancing your QMS, you ensure the highest quality and safety standards for your medical devices. This commitment extends beyond certification, safeguarding patient well-being and your organization’s reputation.
At SQC Certifications Service Pvt. Ltd, we’re your trusted partners on the journey to ISO 13485:2016 Certification. Together, we’ll elevate your organization’s commitment to medical device quality, safety, and compliance. Join us in ensuring that your products make a positive impact on healthcare around the world.
Our Service
Interested in getting ISO certified or need ISO training services?
Partner with one of India’s top ISO certification bodies to easily and affordably achieve ISO certifications.
FAQs
ISO 13485:2016 Certification is a globally recognized standard for Quality Management Systems (QMS) specific to the medical device industry. It's crucial because it ensures that your organization meets stringent quality and safety requirements, enhances patient safety, and helps you comply with regulatory standards.
ISO 13485:2016 Certification is suitable for organizations involved in the design, development, manufacturing, distribution, and servicing of medical devices. This includes medical device manufacturers, suppliers, and related service providers.
The certification process involves a series of steps, including gap analysis, QMS development, implementation, internal audits, and a final external audit by a certification body. Successful certification demonstrates that your organization complies with ISO 13485 standards.
Yes, ISO 13485:2016 Certification is applicable to organizations of all sizes, from small start-ups to large multinational corporations. It offers a flexible framework that can be tailored to meet the specific needs of different organizations in the medical device industry.
Yes, ISO 13485:2016 Certification is designed to help organizations comply with medical device regulations and standards, making it easier to navigate regulatory requirements in various regions.
